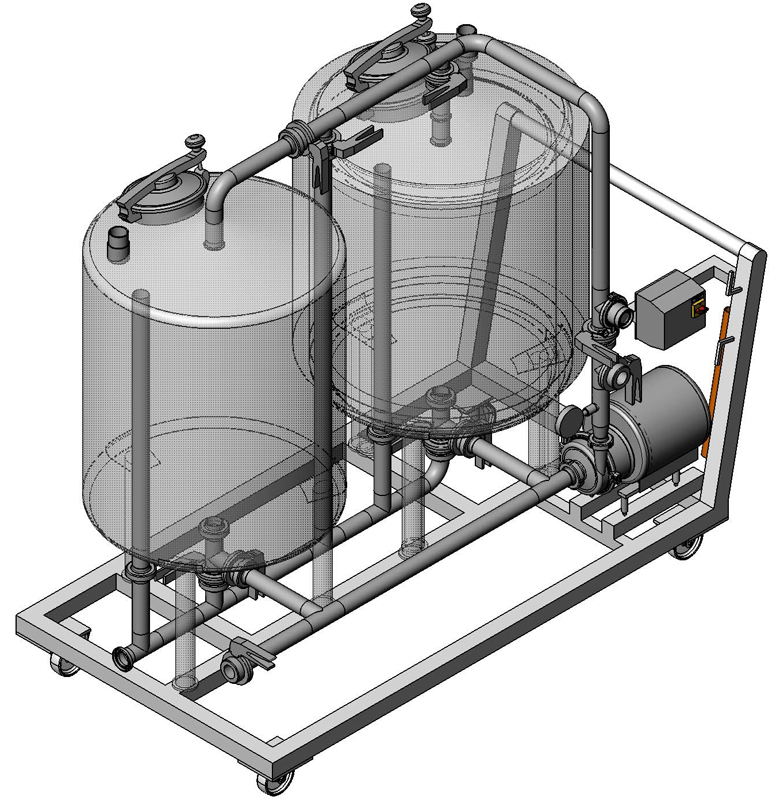
CIP Manual and Automated
Hygiene is an essential part of the processes of the food processing, cosmetics, pharmaceutical industries as a correct cleaning of all the elements is required (tanks, pipes, pumps, etc.). We offer automated CIP units, correctly selected and customized to guarantee a controlled cleaning and efficiency without having to disassemble the plant.
Innovative CIP concepts meet comprehensive high standards. We guarantee product safety at every point of the process. Every upgrade is adapted to individual local conditions and customer requirements and leads to noticeable savings.
Individual consulting and customer-specific configurations aim at low investment costs, fast commissioning and short downtimes. Complete integration into the existing process control system ensures automatic and trouble-free cleaning and higher economic efficiency at the same time.
Today, modern Production Processes cannot be imagined without CIP systems. In view of rising costs, particularly for fresh and waste water, CIP systems have recently been in the focus of interest to identify possible saving potentials. However, for reasonable savings by means of equipment optimisation certain criteria have to be met. When it comes to the cleaning of equipment, vessels and pipes in a brewery, the major criterion can only be perfect cleanliness.
After preparation of the concept and order placement, project execution and successful commissioning takes just a few weeks.